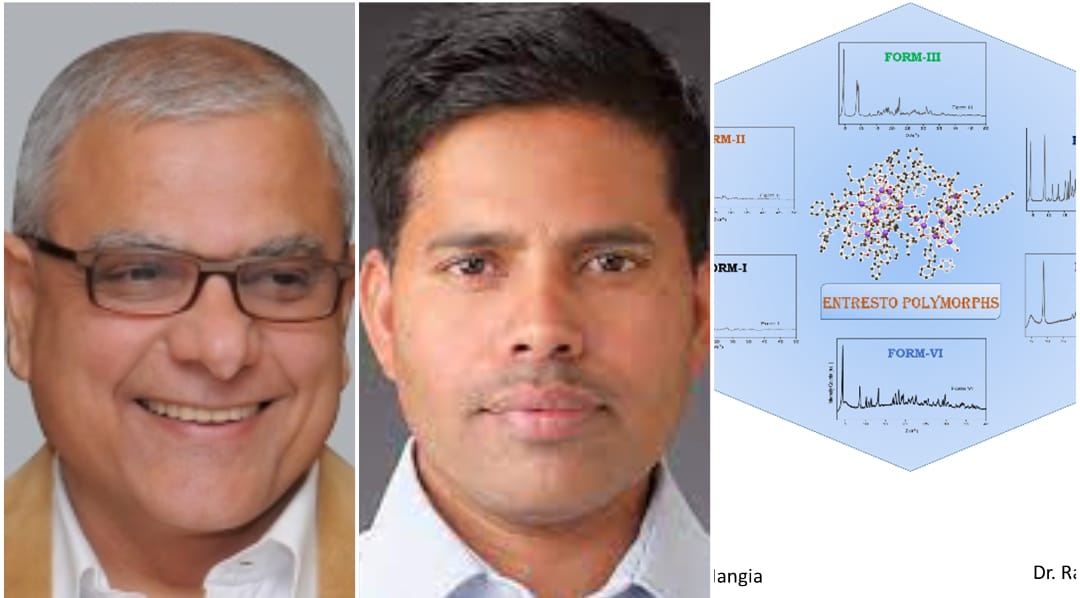
Pune, 23rd September 2022: CSIR-National Chemical Laboratory (CSIR-NCL), Pune as part of a Mission Mode program funded by the Council of Scientific and Industrial Research (CSIR), initiated research on novel crystalline hydrate forms and polymorphs of Entresto in 2018. Prof. Ashwini Kumar Nangia, then Director of CSIR-NCL, and Dr. Rajesh G. Gonnade, Chief Scientist from the Physical and Materials Chemistry Division, led the project.
The comprehensive study has appeared in the Royal Society of Chemistry journal CrystEngComm, which deals with crystal engineering and drug polymorphs. The research team has successfully characterized and identified six different crystalline forms of Entresto, with varying amounts of water content and containing the active pharmaceutical ingredients (APIs) valsartan and sacubitril in their anionic state with bonded sodium cations. The different hydrated forms of Entresto have 2.0-3.2% water and exhibit additional stability to temperature and moisture, which is vital for their long-term storage, shelf-life, and drug bioavailability. This paper is a first of its kind wherein investigators have shown that large supramolecular drug complexes pose unique challenges in the characterization of polymorphs and hydrate structures by powder X-ray diffraction, thermal measurements, and spectroscopic techniques. The next task for the team will be to understand the molecular packing arrangement and hydrogen bonding with water by determining its crystal structures.
Prof. Gautam R. Desiraju, Emeritus Professor, IISc, Bengaluru and former president of International Union of Crystallography, has commented on the significance of this novel disclosure, saying that these results are important because it has come at the intersection of cutting-edge science, legal questions, and commercial viability. He added that utmost scrutiny from various quarters is needed to safeguard IP from Indian labs and institutions; India is at the forefront of international crystal engineering research.
Entresto is the world’s top-selling drug launched for the treatment of chronic heart failure in advanced critical patients, approved by the US FDA in 2015. It is different from other drugs having a multi-drug supramolecular complex of sacubitril and valsartan as a trisodium hemi-pentahydrate salt-cocrystal. Most drugs in the market are single drug molecules, while others are fixed dose combinations (FDCs) or administered as a combination of multiple drugs (cocktail dosage) for treatment. Entresto is a first-in-class drug designed and developed using crystal engineering principles, supramolecular synthons and pharmaceutical cocrystals published in the academic literature starting from the early 2000s and patents filed in subsequent years. A drug can exist in the solid state in more than one crystal form, referred to as polymorphs. These are important for tablet oral formulation and its bioavailability properties, such as solubility, permeability, and adsorption in the body. Entresto is different from other drugs in other aspects too.
For example, the cocrystal-salt complex’s crystal structure comprises six sacubitril and valsartan molecules, each in their anionic form, along with 18 penta- and hexa-coordinated sodium cations and 15 water molecules, thus providing a molecular formula of C288 H330 N36 O48 Na18 15H2O, of molecular weight 5748. On the other hand, most drugs have a molecular weight of less than 500.





